Bundle

Acids, Bases & Buffers (OCR)
10 Full Lesson Bundle + BONUS lesson on Acids, bases & buffers. This bundle covers the OCR A Level Chemistry specification. Please review the learning objectives below.
Lesson 1: Bronsted-Lowry Acid and Bases
To describe the difference between a BrØnsted Lowry acid and base
To identify conjugate acid-base pairs
To explain the difference between monobasic, dibasic and tribasic acids
To understand the role of H+ in the reactions of acids with metals and bases (including carbonates, metal oxides and alkalis), using ionic equations
Lesson 2: Strong Acids & The pH Scale
To calculate the pH of a strong acid
To convert between pH and [H+(aq)]
To apply the relationship between pH and [H+(aq)] to work out pH changes after dilution
**Lesson 3 - The Acid Dissociation Constant **
To understand the acid dissociation constant, Ka, as the extent of acid dissociation
To know the relationship between Ka and pKa
To convert between Ka and pKa
Lesson 4- pH of weak acids
To recall the expression of pH for weak monobasic acids
To calculate the pH of weak monobasic acids using approximations
To analyse the limitations of using approximations to Ka related calculations for ‘stronger’ weak acids
Lesson 5 - The ionic product of water
To recall the expression for the ionic product of water, Kw (ionisation of water)
To calculate the pH of strong bases using Kw
To apply the principles for Kc, Kp to Kw
Lesson 6-9 - Buffer Solutions (3 part lesson)
Part 1: Explaining How Buffer Solutions Work
To know a buffer solution is a system that minimises pH changes on addition of small amounts of an acid or base
To describe how a buffer solution is formed using weak acids, salts and strong alkalis
To explain the role of the conjugate acid-base pair in an acid buffer solution such as how the blood pH is controlled by the carbonic acid–hydrogencarbonate buffer system
Part 2: Buffer Solution Calculations (Part 1)
To calculate the pH of a buffer solution containing a weak acid and the salt of a weak acid by using the Ka expression and pH equation
To calculate equilibrium concentrations, moles or mass of the components of a weak acid-salt of a weak acid buffer solution
Part 3: Buffer Solution Calculations (Part 2)
To calculate the pH of a weak acid-strong alkali buffer solution
To calculate equilibrium concentrations, moles or mass of the components of a weak acid- strong alkali buffer solution
BONUS Lesson 9 : Revision on Buffer Solutions
To review how to calculate the pH of a buffer solution containing a weak acid and a strong alkali
To review how to calculate the pH of a buffer solution containing a weak acid and the salt of the weak acid
Lesson 10- Neutralisation & Titration Curves
To interpret titration curves of strong and weak acids and strong and weak bases
To construct titration curve diagrams of strong and weak acids and strong and weak bases
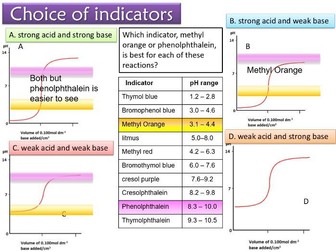
**Lesson 11- pH indicators & Titration Curves **
To explain indicator colour changes in terms of equilibrium shift between the HA and A- forms of the indicator
To explain the choice of suitable indicators given the pH range of the indicator
To describe an experiment for creating a titration curve
Declaimer: Please refrain from purchasing this popular resource for an interview lesson or a formal observation. This is because planning your own lessons including using your own lesson PowerPoints is a fundamental skill of a qualified/unqualified teacher that will be reviewed during these scenarios outlined above

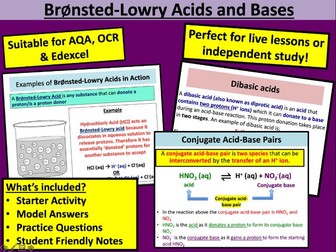
Bronsted Lowry Acid and Bases
A structured KS5 lesson including starter activity, AfL work tasks and main work task all with answers on Bronsted Lowry Acids and Bases
By the end of this lesson KS5 students should be able to:
To describe the difference between a BrØnsted Lowry acid and base
To identify conjugate acid-base pairs
To explain the difference between monobasic, dibasic and tribasic acids
To understand the role of H+ in the reactions of acids with metals and bases (including carbonates, metal oxides and alkalis), using ionic equations
Declaimer: Please refrain from purchasing this popular resource for an interview lesson or a formal observation. This is because planning your own lessons, including using your own lesson PowerPoints, is a fundamental skill of a qualified/unqualified teacher that will be assessed during the scenarios outlined above

Acid base booklet
A booklet covering experiments and worksheets on the main content requirements for the 'acids and bases' topic for AS Chemistry.
Sale

Acids, Bases, and the pH Scale - Worksheet | Printable and Distance Learning
This worksheet is the perfect way for helping your students learn and review Acids, Bases, and the pH Scale.
What is included in this resource?
Printable and editable Student Worksheet (PDF and Word document)
Paperless digital version for use in Google Drive (Prepared with Google Slides)
Complete Answer Key

A level chemistry revision: acids, bases and buffers
Presentations and worksheets to revise A level chemistry acids, bases and buffers topics. This was designed for the OCR A level chemistry A course. It contains a worksheet summary of the topic for students to add details to as well as some practice calculations with answers.
undefined

Acid and base revision broadsheet
Broadsheet made for the AQA scheme but will work for OCR. Revision broadsheet that covers: Acids, bases, strong/weak acids, ions, neutralisation, equations, soluble salts, insoluble salts.

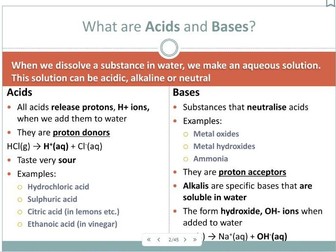
Acids and Bases for AS
A power point to cover 2.1.4 OCR AS Chemistry Acids, bases, alkalis and neutralisation.

Acids and bases
This worksheet and answer sheet is aimed at post 16 chemistry students and covers acids, bases and salts.
Sale

Acid and Bases
A fully detailed lesson plan on Acid and Bases with starter, main, activity, assessment and plenary

ACIDS AND BASES
A lesson where students will investigate the meanings of Arenhuis and Lowry-Bronstead theories.
Students will also calculate the concentration of acids using exam style questions.

A-Level Acids bases and buffers
Two powerpoints for teaching and revising acids bases and buffers. Worked examples of calculations and includes some exam questions. Spec points and exam questions are from AQA but content related to all exam boards.
Please message me with feedback.

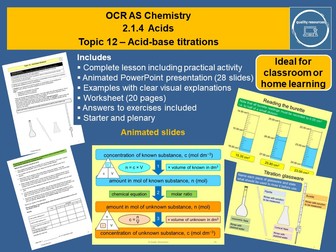
Acid-base titrations OCR AS Chemistry
This complete year 12 resource on acid-base titrations includes the practical procedure and calculations for titrations as well as details of evaluating experiments. It features a 28 slide interactive PowerPoint that illustrates the concepts in a lively, visual and systematic way. The resource includes a starter, learning checks, clearly explained examples of calculations, a practical activity with evaluation and a plenary. A 20 page worksheet includes a variety of structured and unstructured calculations and answers to all exercises. Ideal for the classroom or blended learning, this resource could be used to present the topic, or for revision, extension or consolidation.
This lesson is part of a series covering the OCR AS Chemistry specification and relates to the following sections:
Module 2 – Foundations in chemistry
Part 1 – Atoms and reactions
2.1.4 – Acids (part)
Content covered:
• Titration and uses
• Standard solution
• Glassware and procedure for titration with detailed hints for technique
• Reading burette
• Recording titration results and calculating the mean
• Titration calculations
• Examples of structured and unstructured calculations
• Revision of calculations involving masses and volumes
• Practical titration activity
• Evaluation of titration experiment
• Uncertainties and calculating % uncertainties
• Procedural errors
Duration: 2 lessons
Please review!
Links
Previous topic: Topic 11 – Acids and bases (free resource)
https://www.tes.com/teaching-resource/acids-and-bases-ocr-as-chemistry-12747201
Next topic: Topic 13 – Redox
https://www.tes.com/teaching-resource/redox-ocr-as-chemistry-12409890
Related topics
Topic 8 − Moles and concentration of solutions
https://www.tes.com/teaching-resource/moles-and-concentration-of-solutions-ocr-as-chemistry-12391026
Topic 9 – Moles and reactions
https://www.tes.com/teaching-resource/moles-and-reactions-ocr-as-chemistry-12404411
Bundle − Moles, masses, concentrations, gas volumes and reactions
https://www.tes.com/teaching-resource/moles-masses-concentrations-gas-volumes-and-reactions-12404451
Sale

Acids and Bases
A fully detailed lesson plan on Acids and Bases (Starter, Main, Activity, Assessment and Plenary)

Acid and Bases
Bronsted-Lowry theory
Acid-Base Reactions
Ethanoic Acid
Conjugate Acid-Base Pairs
Ammonia
Acid Strengths
Acid Dissociation Constant (Ka)
Role of water
Henderson-Hasselbalch Equation

Acids and bases
A double-sided worksheet and answer sheet which is written in American English and aimed at 16+ year old chemistry students studying acids and bases.

Acid and Base
This packet covers the following learning objectives through simplified one-page readings, virtual labs, guided inquiry, researching secondary database etc. Includes a formative after every lesson and guidelines for a project based summative.
Define Arhennius acid and base.
Identify acid and base from their formula.
Describe characteristic properties of acids in their reaction with metals, metal oxides and hydroxides and metal carbonates
Describe the characteristic properties of bases in their reaction with acids and ammonium salts.
Understand the pH scale as a figure expressing the acidity or alkalinity of a solution.
Apply knowledge of indicators (Litmus, Methyl Orange and Universal Indicator) to determine pH of solutions.
Differentiate between strong and weak and concentrate and dilute acids and alkali.
Discuss acid rain and ocean acidification.

AQA Chemistry A Level Acids and Bases
A full set of PowerPoints for AQA Chemistry A Level Acids and Bases

Introduction to Acids and Bases Lesson
Includes starters, PowerPoint, activity cards with different acids and bases, a worksheet to complete with challenge questions, learning checklist to measure progress and plenary to also assess progress. Help sheets for all activities are also included.
Lesson is aimed at high ability GCSE students but could easily be differentiated with the use of the help sheets. Activities could also be completed in competition as engaging revision lesson.
Relevant to KS3 specifications as well as GCSE Physics specifications.

Acids and Bases - Edexcel IGCSE Chemistry
Complete lessons and workbook
Content
Describe the use of litmus, phenolphthalein and methyl orange
to distinguish between acidic & alkaline solutions
Understand how to use the pH scale, from 0–14, to classify
solutions as strongly acidic (0-3), weakly acidic (4-6), neutral (7),
weakly alkaline (8-10) and strongly alkaline (11-14)
Describe the use of Universal Indicator to measure the
approximate pH value of an aqueous solution
Know that acids in aqueous solution are a source of hydrogen
ions and alkalis in an aqueous solution are sources of hydroxide
ions
Know that metal oxides, metal hydroxides and ammonia can be
classified as bases and that alkalis are bases that are soluble in
water
Know that bases can neutralise acids
Understand acids and bases in terms of proton transfer and that
an acid is a proton donor and a base is a proton acceptor
Understand how to use acid-base character of oxides to classify
elements as metals or non-metals
Describe the combustion of elements in oxygen, including
magnesium, hydrogen and sulfur
Describe the reactions of hydrochloric, sulfuric and nitric acids
with:
metals (but not with nitric acid)
bases
metal carbonates
Describe an experiment to prepare a pure, dry sample of a
soluble salt starting from an insoluble reactant
Describe an experiment to prepare a pure, dry sample of a
soluble salt starting from an acid and an alkali
Describe an experiment to prepare a pure, dry sample of an
insoluble salt starting from two soluble reactants

Acids and Bases Task Cards
These task cards are a great way for students to improve their skills and knowledge of acids and bases.
This product contains 24 cards with multiple choice questions about acids and bases. A recording sheet and an answer key are included. Blank cards are also included for questions to be added, if wanted. The same 24 cards are also available as open ended questions.